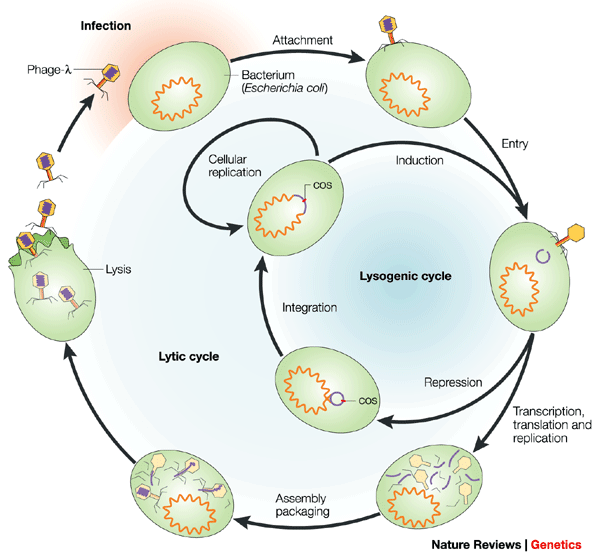
Lambda Phage Structure and Life Cycle
Describe Structure And Life-cycle of lambda Phage
Lambda phage, also known as bacteriophage lambda, is a virus that infects bacteria, particularly Escherichia coli (E. coli). It has a complex life cycle involving both lytic and lysogenic pathways. Here’s an overview of its structure and life cycle:
Structure:
- – Lambda phage has a complex structure. Its head contains the genetic material, which is double-stranded DNA. The head is enclosed in a protein capsid.
- – The tail of lambda phage is used for attaching to the host bacterium and injecting its genetic material into it.
- – Lambda phage also has tail fibers that help it recognize and attach to specific receptors on the bacterial cell surface.
Life Cycle:
- 1. Attachment and Injection: The lambda phage attaches to the surface of the E. coli bacterium using its tail fibers. It then injects its DNA into the bacterial cell through the cell wall and membrane.
- 2. Lytic Cycle (Virulent Cycle):
- Immediate Early Genes: Upon injection, some of the lambda DNA immediately expresses genes that prevent the host from destroying the phage DNA.
- Early Genes: The phage DNA then integrates into the host’s genome. This integrated phage DNA is called a prophage. The prophage replicates along with the host DNA and is passed on to daughter cells during cell division.
- Late Genes: Under certain conditions, such as when the host is stressed, the prophage can excise itself from the host genome and enter the lytic cycle.
- Lysis: The phage then starts replicating its DNA and producing phage proteins. The newly formed phage particles assemble within the host cell. Eventually, the host cell lyses (bursts), releasing the new phage particles, which can infect other bacteria.
- 3. Lysogenic Cycle (Temperate Cycle):
- Instead of entering the lytic cycle, the lambda phage can remain in the lysogenic cycle. In this cycle, the phage DNA is integrated into the host genome as a prophage without causing cell lysis.
- The prophage is replicated along with the host DNA and is passed on to daughter cells during cell division.
- Under certain conditions, such as stress or UV radiation, the prophage can excise itself from the host genome and enter the lytic cycle, leading to cell lysis and the production of new phage particles.
The ability of lambda phage to switch between the lytic and lysogenic cycles allows it to adapt to different environmental conditions and ensure its survival.
Define Immunity Describe In Detail Factors Affecting in innate immune response
Immunity refers to the body’s ability to resist or fight off infections and other harmful pathogens. It can be broadly categorized into two types: innate immunity and adaptive immunity. Innate immunity is the first line of defense against pathogens and is present from birth. It provides immediate, nonspecific protection against a wide range of pathogens.
Innate immune responses are triggered when pattern recognition receptors (PRRs) on immune cells recognize specific molecules that are common to many pathogens, such as bacterial cell wall components or viral nucleic acids. This recognition triggers a cascade of events that lead to the activation of various immune cells and the release of chemical mediators that help eliminate the pathogen.
Factors affecting innate immune responses include:
- 1. Genetics: Genetic factors play a significant role in determining the effectiveness of the innate immune response. Variations in genes encoding PRRs or other immune molecules can affect an individual’s susceptibility to infections.
- 2. Age: The innate immune response tends to be more robust in younger individuals and declines with age. This is one reason why older adults are more susceptible to infections.
- 3. Nutritional Status: Adequate nutrition is essential for a healthy immune system. Deficiencies in certain nutrients, such as vitamin D, vitamin C, and zinc, can impair innate immune function.
- 4. Stress: Chronic stress can suppress the immune system, including the innate immune response, making individuals more susceptible to infections.
- 5. Sleep: Lack of sleep or poor sleep quality can weaken the immune system, including the innate immune response.
- 6. Exercise: Regular moderate exercise can enhance innate immune function, while intense or prolonged exercise can temporarily suppress it.
- 7. Environmental Factors: Exposure to pollutants, toxins, or allergens can impair innate immune function.
- 8. Microbiota: The composition of the microbiota in the gut and other mucosal surfaces can influence innate immune responses.
- 9. Medications: Certain medications, such as corticosteroids or immunosuppressants, can suppress innate immune function.
- 10. Previous Exposures: Previous exposure to pathogens can influence the strength and effectiveness of the innate immune response upon subsequent encounters with the same or similar pathogens.
These factors, among others, can influence the strength and effectiveness of the innate immune response, thereby affecting an individual’s ability to resist infections.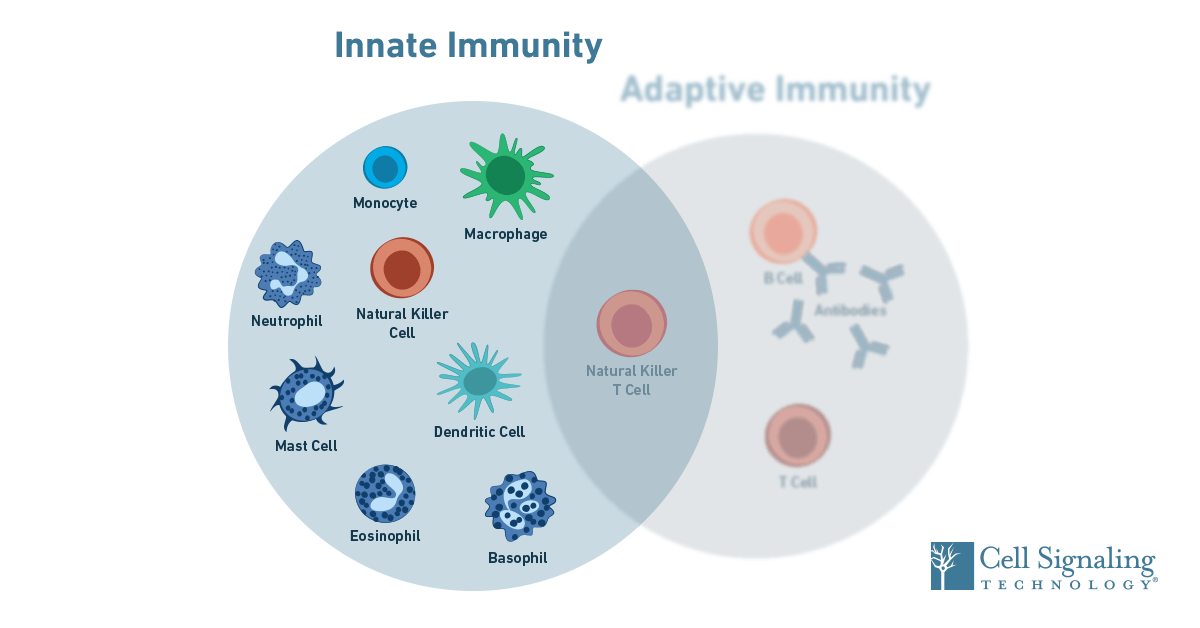
Describe IN Detail Different Classes Of Immunoglobulins
Sure, here’s a detailed description of the different classes of immunoglobulins:
1. IgG (Immunoglobulin G):
- – Structure: IgG is the most abundant class of antibodies in the bloodstream, accounting for about 75% of the total antibodies in the body. It is a monomeric antibody, meaning it consists of a single Y-shaped unit composed of two heavy chains and two light chains.
- – Function: IgG plays a key role in secondary immune responses. It can neutralize toxins, opsonize pathogens for phagocytosis, and activate the complement system.
- – Subclasses: IgG has four subclasses in humans: IgG1, IgG2, IgG3, and IgG4, each with slightly different functions and properties.
2. IgA (Immunoglobulin A):
- – Structure: IgA exists in two forms – monomeric IgA and dimeric IgA. Dimeric IgA is the most common form found in the mucous membranes and secretions such as saliva, tears, and breast milk.
- – Function: IgA plays a crucial role in mucosal immunity by preventing pathogens from attaching to mucosal surfaces and by neutralizing toxins.
- – Location: IgA is predominantly found in the mucous membranes of the respiratory, gastrointestinal, and genitourinary tracts.
3. IgM (Immunoglobulin M):
- – Structure: IgM is a pentameric antibody, meaning it consists of five Y-shaped units linked together by a joining (J) chain. It is the largest antibody in the body.
- – Function: IgM is the first antibody produced in response to an infection and is involved in the primary immune response. It is particularly effective at agglutinating pathogens.
- – Location: IgM is found in the bloodstream and is also expressed on the surface of B cells.
4. IgE (Immunoglobulin E):
- – Structure: IgE is a monomeric antibody similar in structure to IgG but with an additional domain in the heavy chain.
- – Function: IgE is involved in allergic reactions and plays a role in defending against parasitic infections. It triggers the release of histamine and other inflammatory mediators from mast cells and basophils.
- – Location: IgE is found in small amounts in the bloodstream.
5. IgD (Immunoglobulin D):
- – Structure: IgD is a monomeric antibody similar in structure to IgG and IgE.
- – Function: IgD is primarily found on the surface of B cells, where it acts as a receptor for antigen recognition. Its exact role in the immune response is not fully understood.
Each class of immunoglobulin has unique properties and functions, but they all play important roles in the immune system’s ability to recognize and defend against pathogens.
Describe ICTV-Classification Of Viruses
The International Committee on Taxonomy of Viruses (ICTV) classifies viruses based on a hierarchical system that considers their genetic material, replication strategy, morphology, and other characteristics. Here’s a general overview of how viruses are classified by the ICTV:
- 1. Realm: This is the highest taxonomic rank. Viruses are classified into two realms:
- – Riboviria: Viruses with RNA genomes.
- – Duplodnaviria: Viruses with DNA genomes.
- 2. Subrealm: Further categorization based on specific characteristics.
- 3. Kingdom: Viruses are grouped into kingdoms based on their genetic material and replication strategy:
- – Positive-sense ssRNA viruses: These viruses have RNA genomes that can be directly translated by host ribosomes.
- – Negative-sense ssRNA viruses: These viruses have RNA genomes that must be converted into a positive-sense RNA strand before translation.
- – Double-stranded RNA viruses: These viruses have RNA genomes with two complementary strands.
- – Double-stranded DNA viruses: These viruses have DNA genomes with two complementary strands.
- – Single-stranded DNA viruses: These viruses have DNA genomes with one strand.
- – Reverse transcribing viruses: These viruses use reverse transcriptase to convert their RNA genome into DNA before integration into the host genome.
- 4. Phylum: Further classification based on additional characteristics such as genome structure, replication strategy, and morphology.
- 5. Class: Further categorization based on specific features.
- 6. Order: Viruses are grouped into orders based on shared characteristics.
- 7. Family: Viruses are grouped into families based on similarities in genetic content, replication strategy, and morphology.
- 8. Genus: Viruses within a family are grouped into genera based on shared characteristics.
- 9. Species: The most specific level of classification. Viruses within a genus are further divided into species based on distinct biological properties.
Each level of classification is based on specific criteria determined by the ICTV, and the classification scheme is continually updated as new information about viruses becomes available.
Describe in Detail Life Cycle Of Lambda Phage
The lambda phage, or bacteriophage lambda, is a virus that infects bacteria, specifically Escherichia coli (E. coli). Its life cycle can be divided into two main stages: the lytic cycle and the lysogenic cycle. Each cycle has distinct phases that contribute to the virus’s replication and propagation.
1. Attachment and Entry (Lytic and Lysogenic Cycles)
- – Lytic Cycle: The lambda phage attaches to the surface of the host bacterium using its tail fibers. It then injects its genetic material, which is a linear double-stranded DNA molecule, into the bacterial cell.
- – Lysogenic Cycle: Similar to the lytic cycle, the lambda phage attaches and injects its DNA into the host cell. However, in this cycle, the phage DNA integrates into the bacterial chromosome, becoming a prophage.
2. Integration (Lysogenic Cycle)
- After integration, the lambda DNA is known as a prophage. It becomes a part of the host cell’s genome and is replicated along with the bacterial DNA as the cell divides.
3. Lysogeny (Lysogenic Cycle)
- The bacterial cell harboring the integrated prophage is called a lysogen. During lysogeny, the prophage remains dormant, and the host cell continues to grow and divide normally.
4. Induction (Lysogenic Cycle)
- Under certain conditions, such as UV radiation or other stressors, the prophage can be induced to excise itself from the bacterial chromosome and enter the lytic cycle.
5. Replication and Transcription (Lytic Cycle)
- Once inside the host cell, the lambda phage’s DNA is replicated, and its genes are transcribed to produce viral proteins and new copies of the phage genome.
6. Assembly (Lytic Cycle)
- The newly synthesized viral components are assembled to form complete lambda phage particles, including the phage DNA packaged inside the protein coat.
7. Lysis and Release (Lytic Cycle)
- Enzymes produced by the lambda phage cause the bacterial cell to lyse, or burst open, releasing the newly formed phage particles. These particles can then infect neighboring bacterial cells and initiate new infection cycles.
8. Re-infection or Lysogeny (Lytic Cycle)
- The released phage particles can infect new bacterial cells, starting the lytic cycle again. Alternatively, if the conditions are favorable, the phage DNA can integrate into the new host chromosome, entering the lysogenic cycle.
The choice between the lytic and lysogenic cycles is controlled by the genetic regulatory mechanisms of the lambda phage, which can sense environmental conditions and determine the most advantageous course of action for the virus.